Referenced literature:
Back to the future: Epigenetic clock plasticity towards healthy aging
Genome Biology: DNA methylation age of human tissues and cell types
DNA methylation GrimAge strongly predicts lifespan and healthspan
Clonal hematopoiesis associated with epigenetic aging and clinical outcomes
The Lancet Healthy Longevity: Inclusion of epigenetic age acceleration in oncological trials
Dear readers,
Since the dawn of time, great thinkers have pondered life’s big questions. How did life begin on earth? How many different species of plants and animals exist? Who or what do we share this universe with? Unable to answer any of these, this newsletter will focus on something of lesser importance, epigenetic clocks.
Epigenetics refers to the features that alter genetics without actually changing the sequence of our genome. This usually takes the form of molecules (such as methyl or acetyl groups) that bind to different regions of the genome, or changes in the way that DNA is folded around the histone proteins that keep it together. These modifications have downstream effects on gene expression, or the degree to which our genes are produced. Because of this, the epigenome can play a critical role in both disease and the body’s response to disease. For example, it has been well documented that in many cancers, the epigenome undergoes major changes in the methylation groups that bind to the DNA, leading to dysregulation of gene expression and eventually leading to the progression and acceleration of disease.
In 2013, fellow UCLA Bruin Steve Horvath published the seminal paper on epigenetic clocks, introducing the concept of “epigenetic age”, and hypothesizing that it is closely tied to, well, aging. By analyzing the state of many epigenetic marks in the genome, Horvath found that they could be combined to form a score that reflects the “age” of a biological system. Basically, it seems as if these modifications and alterations that sit atop our DNA have something to do with the conditions of our cells, and therefore, of us. Alright Horvath, pretty cool.
So how is it calculated? Briefly, Horvath looked at data from over 20,000 CpG sites (where a cytosine sits next to a guanine in our DNA, bonded by a phosphate, this is where methyl groups like to bind), across many different tissues. Using a penalized regression model, he identified the 353 CpG sites across tissues that are most informative for predicting age. And with just 353 CpG sites, Horvath’s predictor of age using epigenetics had a 0.96 correlation to actual age of patients in a test set.
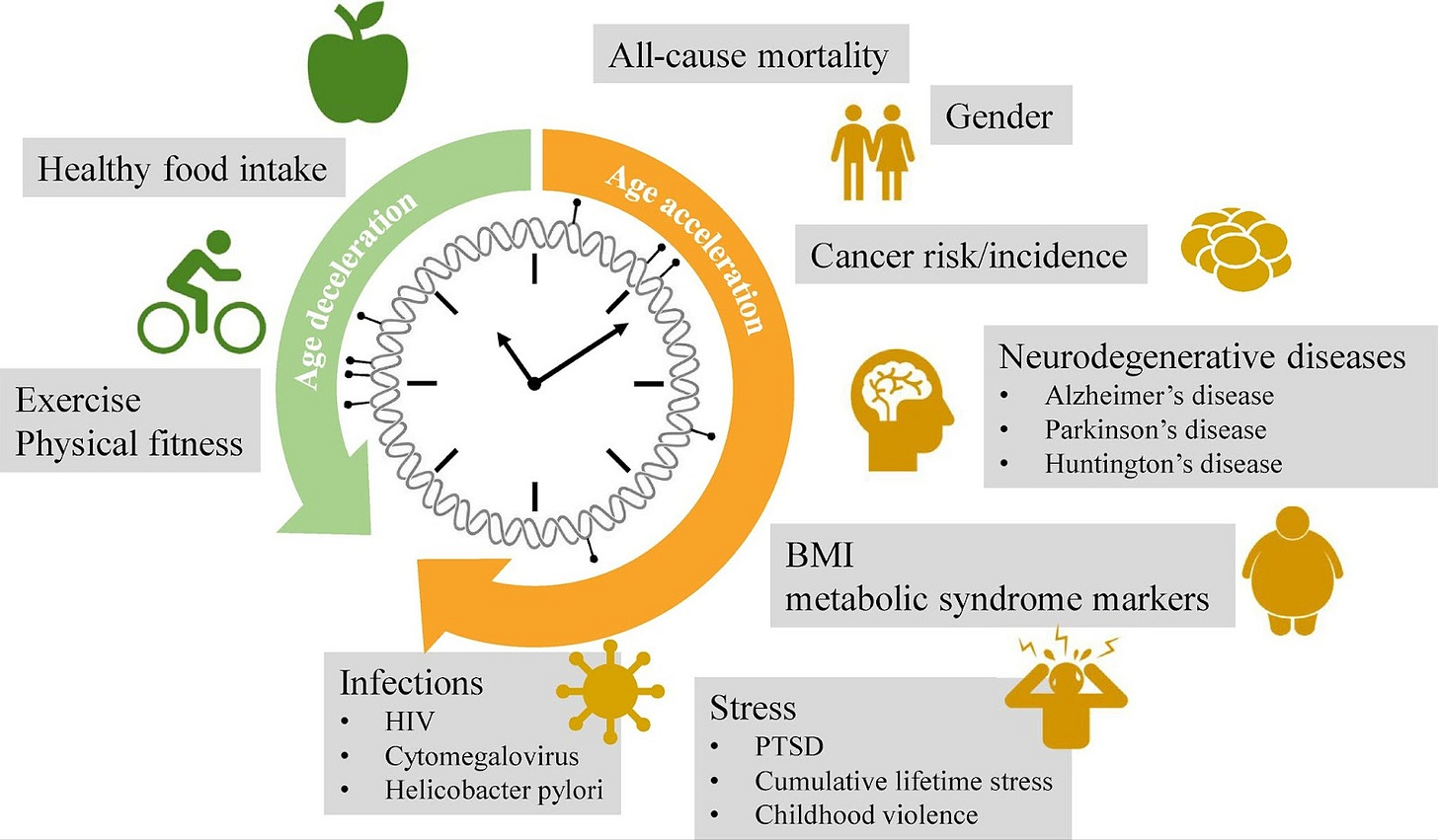
Since the initial development of the epigenetic clock, Horvath and others have worked on variations of the clock that specialize in the prediction of “biological age” as an indicator of disease risk and overall health. These variations have been used as biomarkers to estimate disease susceptibility and mortality risk. For example, Horvath has published two other epigenetic clocks, called GrimAge and PhenoAge, that are geared towards measuring disease risk. Interestingly, while negative lifestyle factors such as smoking and inactivity can increase one’s predicted “biological age”, positive lifestyle factors such as healthy diet and exercise will decrease the predicted “biological age”. In a review from 2018, authors Ken Declerck and Wim Vanden Berghe explore the ideas of how the interplay of genetics and environment can impact our epigenetics, and how this may influence the rate at which we age. Below, I’m including a figure from that review explaining how our lifestyle habits contribute to acceleration or deceleration of epigenetic age, easily a top three figure featured on this newsletter.
To date, there have been plenty of applications in clinical research demonstrating possible utility of these epigenetic clocks. A 2021 study found an association between accelerated epigenetic age and clonal hematopoiesis of indeterminate potential (CHIP), a precursor state for several blood cancers. In a 2023 article in the Lancet, authors advocate for using epigenetic clocks in clinical settings to understand age acceleration in oncological settings.
However, for the time being, even Horvath himself warns against the clinical utility of epigenetic age, saying “People shouldn’t think that epigenetic age replaces standard clinical biomarkers [such as blood pressure or glucose levels]”. Horvath goes on to describe potential uses for epigenetic clocks in the future, such as risk stratification, or assigning medication according to a person’s epigenetic age.
While we are not quite there yet, I’ll tell you where we indeed are: DNA methylation based epigenetic clocks - for your dog. Embark (incredible name) offers a service that for just $160, you can get an estimate of your dog’s birthday, derived from epigenetics. The Embark Dog Age Test is based off of a published 2022 Horvath epigenetic age estimator, in which the authors manage to generate a single epigenetic clock that applies to both dogs and humans.
I ask for your forgiveness for not getting to any of those big questions from the beginning of the newsletter and thank you for reading this far despite the omission. With brevity in mind, I’ve left quite a bit out about this topic, but left you with some literature at the top of this note to guide your journey in learning more.
Have a safe and pleasant weekend :)
Tommer


It would be interesting to explore business use-cases and opportunities for this type of research... such as personalized healthcare solutions. The medical industry is always needing of innovation and disruption